Weatherability Evaluation
Electrochemical Measurement/Analysis of Corrosion Mechanism
Electrochemical Measurement
JFE-TEC evaluates corrosion characteristics based on measurements of the values of electrochemical characteristics.
Corrosion occurs by an electrochemical reaction, in which an anode (positive electrode) is oxidized (losses electrons) and a cathode (negative electrode) is chemically reduced (received electrons). Therefore, it is possible to evaluate corrosion characteristics and corrosion behavior by performing an electrochemical test and measuring the characteristic values. Electrochemical measurement is one effective analytical technique for corrosion investigation.
Electrochemical Measurement Method
-
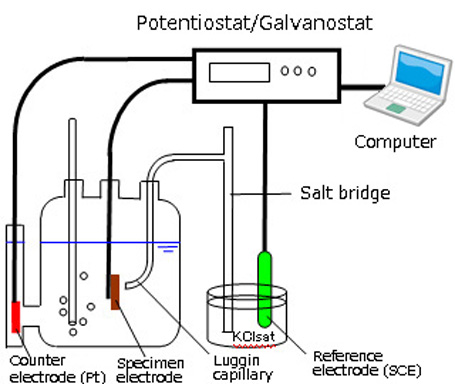
In electrochemical experiments, an electrochemical reaction is caused in a sample in a solution in a cell, and the electrical response is measured. For example, if a specimen of iron, steel or some other metal material is immersed in an aqueous solution, an oxidation-reduction reaction will occur at the specimen surface due to dissolved oxygen and/or ions in the solution, and as a result, its electrical potential is determined. The natural electrode potential ( = corrosion potential = open circuit potential) can then be measured by measuring this potential against that of a reference electrode as a standard.
In general electrochemical measurements, three electrodes are immersed in a test solution (electrolyte). These three electrodes consist of a working electrode (WE), which is the sample material being tested, a counter electrochemical (CE) for passing a current, and a reference electrode (RE), which serves as a standard.
-
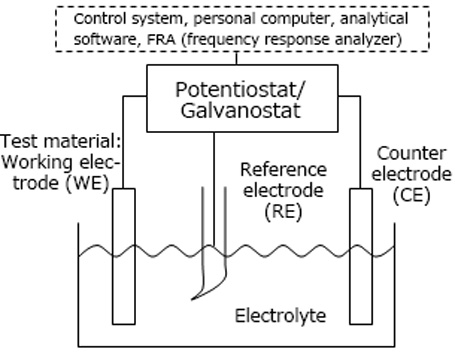
Model diagram of an
electrochemical measurement system
DC Polarization Measurement Method
The potential-current curve is measured by measuring the current when a potential is applied by a potentiostat, or by measuring the potential when a current is passed with a galvanostat. (Corrosion current measurement, etc. by anodic polarization curve measurement, cathodic polarization curve measurement, galvanostatic electrolysis, controlled potential electrolysis, chronometry, voltammetry, Tafel plot method)
AC Impedance Method
Because corrosion is an electrochemical reaction, it can be considered to be an equivalent circuit of an electric circuit consisting of a condenser and resistance. In the alternating current (AC) impedance method, the impedance as an electric circuit is measured from the response signal when a very small AC signal is applied by a frequency response analyzer (FRA). The Cole-Cole plot (Nyquist diagram) or Bode diagram, etc. is analyzed by the software of the electrochemical measurement system, and the quantitative parameters of corrosion characteristics are calculated.
Applications of Electrochemical Measurement
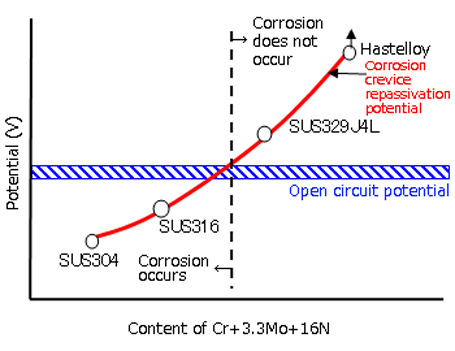
Electrochemical investigations are not limited to the basic condition of an aqueous solution, but can also be performed for states and conditions simulating the actual environments at specific sites. JFE-TEC can perform a diverse range of electrochemical measurements at the request of the client, including measurement of corrosion crevice repassivation potential (ER,CREV), measurement of pitting potential, measurement of electrochemical potentiokinetic reactivation ratio (ERP), measurement of hydrogen diffusivity (hydrogen diffusion coefficient), etc.
Applications of Electrochemical Measurement
JFE-TEC performs electrochemical measurements in response to the client's request. We can perform a wide range of evaluations, from measurement of the values of electrochemical characteristics to evaluation of the corrosion properties and corrosion behavior of materials.
Examples of Electrochemical Measurements
-
- Measurement of electrochemical potentiokinetic reactivation ratio (ERP) → Investigation of susceptibility to intergranular attack of stainless steel
Measurement of polarization curve in 0.5M sulfuric acid + 0.01M potassium thiocyanate solution - Measurement of corrosion crevice repassivation potential (ER,CREV), measurement of pitting potential
- Corrosion monitoring materials of waste incinerator by impedance measurement
- Measurement of "grooving corrosion sensitivity" of electric resistance welded (ERW) pipes by controlled potential electrolysis
- Measurement of corrosion potential of concrete reinforcing steel
- Measurement of corrosion current (icorr) of plain carbon steel for piping use by Tafel plot method
- Electrochemical analysis of galvannealed steel sheets
- Investigation of passivation and stress corrosion cracking of corrosion-resistant materials
- Investigation of electrochemical characteristics of chemical conversion treated materials
- Electrochemical study of corrosion problem in stainless steel
- Electrochemical investigation of cause of corrosion accident trouble in water treatment equipment
- Measurement of electrochemical potentiokinetic reactivation ratio (ERP) → Investigation of susceptibility to intergranular attack of stainless steel
-

Programmed control electrochemical
measurement device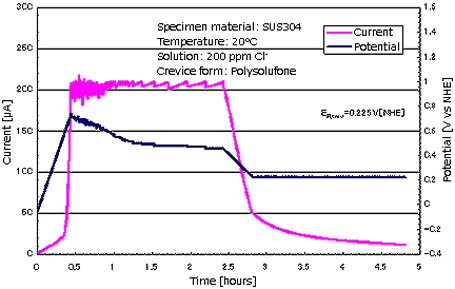
Measurement of corrosion crevice
repassivation potential