Evaluation of Medical Equipment/Implant Materials
MR Compatibility Evaluation Tests Based on ASTM Standards
Evaluation of the effect of MRI (Magnetic Resonance Imaging) Examination on Implanted Medical Devices
Background of MR Compatibility Evaluation Tests Based on ASTM Standards
In recent years, the chances for patients with stents or other implanted medical devices to receive examinations by MRI (magnetic resonance imaging) have increased. From the viewpoint of medical safety, this has heightened the necessity of assessments of the safety of implanted medical devices in MRI examinations. Safety in the MRI environment is called " MR compatibility. "
In the notification " Standard for approval of clips for arteriovenous malformation surgery " dated March 25, 2008, Japan's Ministry of Health, Labour and Welfare requires a response to MR compatibility, for example, requiring an evaluation of the effects of MRI as an attachment to approval applications, etc. At present, there is no JIS standard for assessment of MR compatibility in Japan, but assessment methods for various types of compatibility have been established in ASTM standards.
Outline of MR Compatibility Evaluation Tests Based on ASTM Standards
JFE-TEC evaluates MR compatibility for 4 test items in line with the following ASTM standards.
-
■ Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment (ASTM F2052)

Measurement of the force of attraction of an object by MR equipment (i.e., magnetically induced displacement force) due to the interaction of the magnetic susceptibility of the object and the gradient of the magnetic flux density of a static magnetic field.
-
■ Standard Test Method for Measurement of Magnetically Induced Torque on Medical Devices in the Magnetic Resonance Environment (ASTM F2213)

Measurement of the force causing rotation of an object (i.e., magnetically induced torque) due to interaction of the magnetic bipolarity of the object and the magnetic flux density of a static magnetic field.
-
■ Standard Test Method for Measurement of Radio Frequency Induced Heating On or Near Passive Implants During Magnetic Resonance Imaging (ASTM F2182)
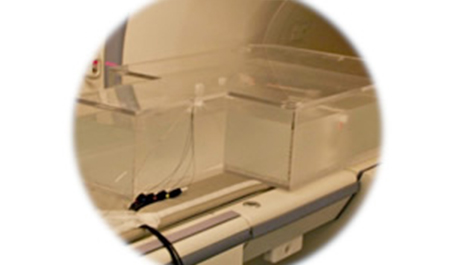
Measurement of the Joule heating of an object by an induced current due to the interaction between the conductivity of the object and a radio frequency (RF) electromagnetic field.
-
■ Standard Test Method for Evaluation of MR Image Artifacts from Passive Implants (ASTM F2119)
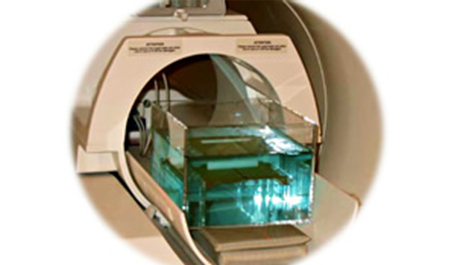
Measurement of image artifacts occurring as a result of disturbance of a magnetic field by the interaction of the magnetic susceptibility of an object and a static magnetic field and a gradient magnetic field, the eddy current field due to the interaction of the conductivity of an object and a gradient field, and the interaction of the conductivity of an object and a RF magnetic field, etc.
We can also prepare test reports in English and a number of other languages!
In addition to Japanese, we can also provide test reports in accordance with ASTM, ISO and other standards in English.
We can also provide expert translations in many other languages. Please discuss your language needs with us when making an inquiry or request.


